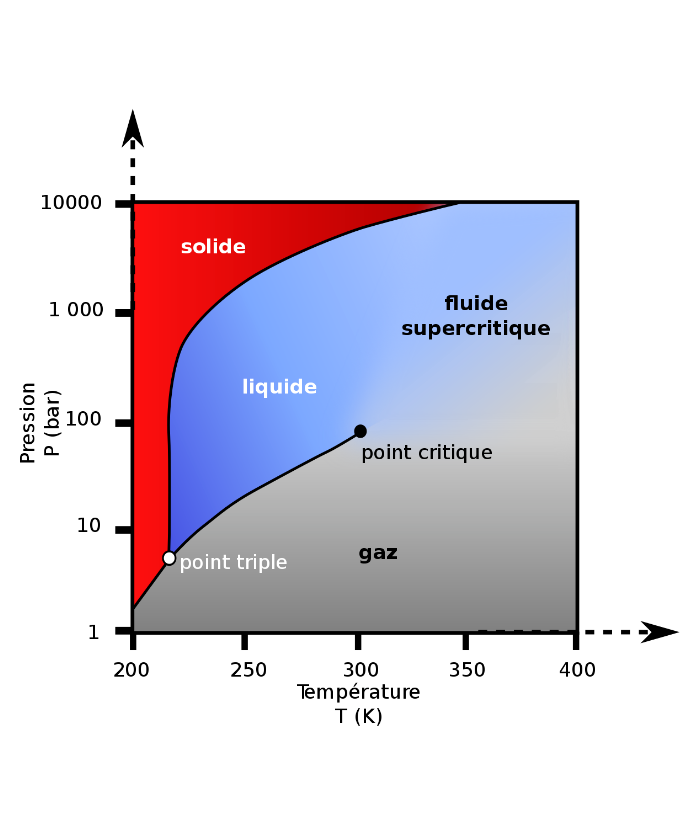
Unique properties
Under specific pressure and temperature conditions, fluids have unique physicochemical properties offering a density close to that of liquids and a viscosity close to that of gases called supercritical state.
In this unique form, supercritical CO2 has a solvent power with variable geometry allowing the efficient extraction of many molecules or compounds depending on their chemical nature thanks to increased solubility and a high diffusion coefficient in the material.
Due to its supercritical conditions of low pressure and temperature, its abundance and its absence of toxicity, supercritical CO2 extraction is today one of the most efficient and environmentally friendly technologies.
Supercritical water offers solvation properties close to those of organic solvents and capable of solubilizing organic compounds insoluble in water in the liquid state. These very specific properties open the way to targeted applications, particularly in the field of waste treatment or organic effluents. Supercritical water can also be used as a reaction medium for hydrothermal oxidation or gasification processes